I. INTRODUCTION
Here we report several case studies on contaminant monitoring in plasma of human and domestic animals from Germany, Italy and the USA using effect based bioassays. There is a strong need to include in the legislation alternative and cost-effective methods that can help to discover possible effects caused by complex mixtures of (known/unknown) chemical s/pharmaceuticals to humans and domestic animals. We will show case studies in which several AOP-based bioassays for e.g. labile and stable AhR agonists, endocrine disruption, genotoxicity and oxidative stress have been used for blood plasma monitoring to evaluate passible effects of complex mixture of pollutants in human and domestic animals. Cell-based screening technologies such as a panel of CALUX® bioassays have shown already several times their usefulness or screening of dioxins in feed and food as well as for human and environmentalmonitoring [1, 2, 3, 4], In this abstract, we present three case studies in Germany, Italy and the USA.
- Germany (2017): Following 2 positive free range chicken eggs samples (DR CALUX®: 6.5 and 7.1; GC/HRMS: 10 and 8.7 pg PCDD/PCDF/dl-PCB-TEQ/g fat) around an industrial landfill in the city of Kamp-Lintfort (situated in the German state of North Rhine-Westphalia) also blood plasma samples in the same region from 86 neighbours have been monitored for dioxins and other dioxin-like compounds by DR CALUX®.
- Italy (2016): Blood plasma samples from 125 humans, buffalos, sheep, dogs and horses living in a from fire with industrial waste contaminated area as well as from two other areas nearby, but not suspected for such pollutions sources, in the wider area of Napoli have been monitored for dioxins/other dioxin-like compounds by DR CALUX®, PAH-like compounds by PAH-CALUX®, for estrogenic-like compounds by ER CALUX®, for genotoxic compounds by p53 CALUX® and for oxidative stress by Nrf2 CALUX®.
- USA (2016): Blood plasma of 301 horses from 33 farms phenotyped for Equine Metabolic Syndrome (EMS) have been analysed by DR CALUX® (for dioxin-like compounds) and ER CALUX® (for estrogenic-like compounds).
II. MATERIAL AND METHOD
* Blood plasma from humans and domestic animals: All blood samples were collected locally, plasma was obtained and send in frozen conditions to BDS and analyzed according to the standard procedures of the CALUX® methods from BDS.
* DR CALUX® bioanalysis: 1-2 gram human plasma was extracted by shake-extraction using hexane: diethyl ether (97:3 v/v) as extraction solvent. Extracted fat is used for clean-up on an acid silica column (20% and 33% H2SO4), topped with sodium sulfate. Cleaned extracts are dissolved in DMSO (8 µL). The DR CALUX bioassay is performed under ISO 17025 accreditation and followed the standard protocols therefore. After 48h incubation time of TCDD and, the plasma samples, the samples were measured by a luminometer (Mithras, Berthold Technologies, Germany). For each set-of samples a blood reference material of BDS was added (mean DR CALUX results: 43 pg total- PCDD/F/dl-PCB-TEQ/g fat; GC/HRMS analysis by both Eurofins-GfA and by MAS did show similar results with both a value of 41 pg total-PCDD/F/dl-PCB-TEQ/g fat).
* PAH, ERα, P53 (+/-S9) and Nrft CALUX®: 0.5 - 1 mL of human plasma is used for extraction for all CALUX® bioassays. Following addition of water, sodium phosphate buffer, K2CO3/KHCO3 buffer and methyl-tertiary butyl ether (MTBE) or ethyl acetate, the plasma is shake-extracted (vortex) for 2 minutes and centrifuged. The extract is re-dissolved in 40 - 80 µL of DMSO. The ER, P53 (+/-S9) and Nrf2 CALUX® bioassays are performed using stably transfected human U2-OS cell lines; PAH CALUX analyses are performed using the stably transfected rat hepatoma H4IIE. After 24 h (PAH CALUX: 4h), the medium is removed, and cells are lysed in 30 pL triton-lysis buffer and measured for luciferase activity using a luminometer (Mithras, Berthold Technologies, Germany) for 0.1 min/well.
III. RESULTS AND DISCUSSION
The results of the first case (Germany 2017) in human blood plasma samples from 86 neighbours living near an industrial landfill in the city of Kamp-Lintfort monitored for dioxins and other dioxin-like compounds by DR CALUX® are presented in table 1 and figure 1. Figure 1 shows all our 86 DR CALUX results lined up with increasing concentrations. Here 95% of all DR CALUX® results (95% percentile level; “end of the vertical hockey stick”) are below 68 pg TEQ/g fat. Such biomonitoring equivalents (BE) for dioxins and dioxin-like compounds are reported from different agencies in the same range between 15-74 pg TEQ/g lipid. The 5% percentile level of our study here of 12 pg TEQ/g fat is also in the same range as an earlier proposed ATSDR minimal risk level (MRL) for dioxins of 15 pg TEQ/g lipid [5]. Here it is suggested to use such BE values for evaluation of biomonitoring data for dioxins in the context of existing risk assessments and for prioritization of the potential need for additional risk assessment efforts for dioxins5.
Table 1 and figure 1
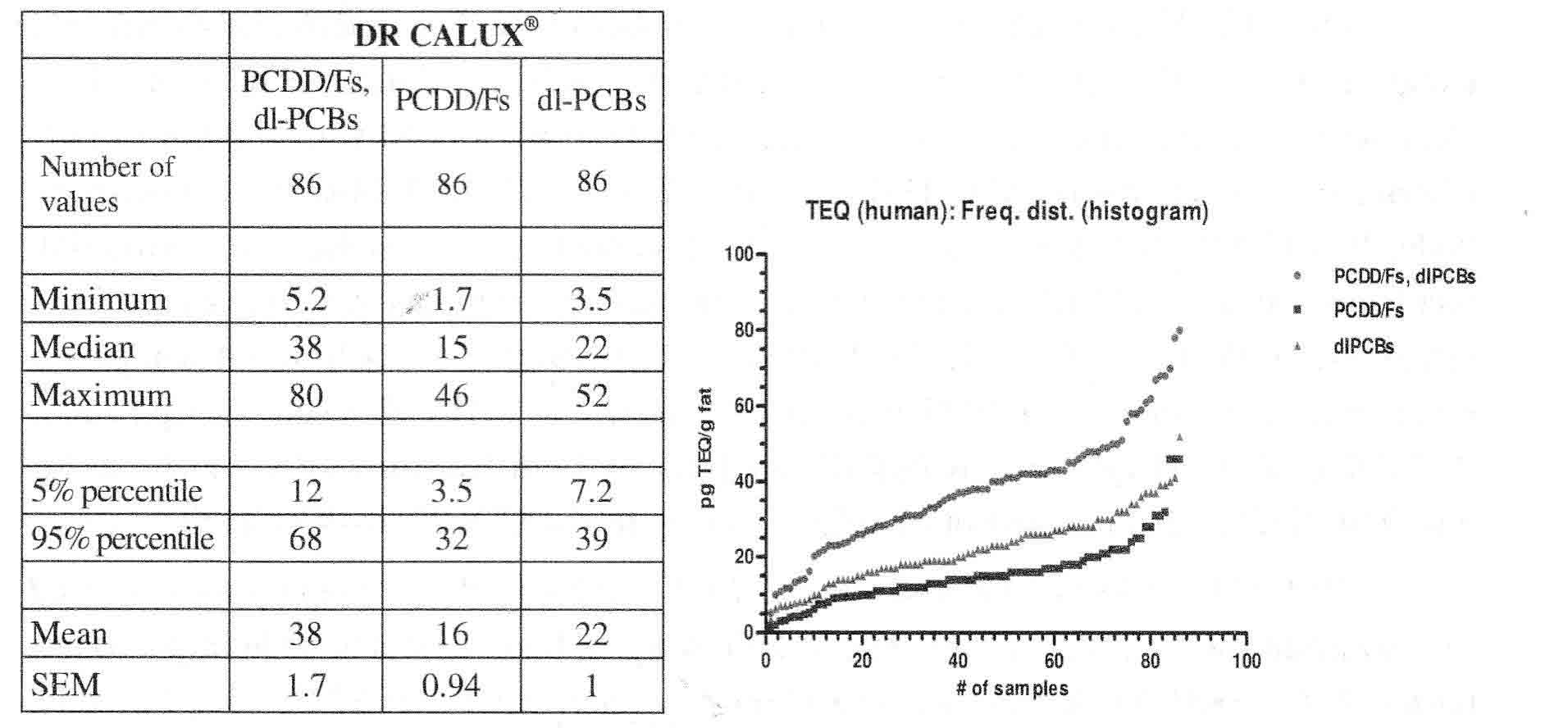 |
Minimum, median, maximum, 95%/5% percentile and mean value of the sum of PCDD/PCDF/dl-PCB/dl-compounds-BEQ, PCDD/F-BEQ and dl-PCB/other dl-like compounds data analysed by DR CALUX® from the human plasma samples from 86 neighbours living near an industrial landfill in the city of Kamp-Lintfort.
The 2nd European case (Italy, 2016) reports about several areas in Italian’s Campania Region using environmental and biological markers, in order to understand pollution of regional area. Three areas have been identified from preliminary analyses: high, medium and low environmental impact. Untamed animals (buffaloes, horses and sheep) and sessile organism (humans, dogs) were be considered for the sampling, from several locations in the Campania region, Italy (e.g. Avellino, Salerno, Caserta and Napoli area). Here in a first pilot study, blood plasma samples have been analyzed for toxic equivalents of dioxin/dl-PCBs (by DR-CALUX®), estrogens (ER-CALUX®), PAHs (by PAH-DR-CALUX®), genotoxic compounds (by p53-DR-CALUX®) and oxidative stressors (by Nrf2-CALUX®). In most samples measurable DR- (e.g. 95% percentile level of 160 pg TEQ/g fat for humans and 180 pg TEQ/g fat for horses, see table 2), ER- (e.g. mean level of 41 pg E2/mL human plasma; see table 3) and PAH- (e.g. mean level of 7.7 µg BaP-EQ/g fat human plasma; see table 3) CALUX levels were found, while oxidative stressors (Nrf2 CALUX®) and genotoxic effects (p53 CALUX®) were below the limit of quantification (LOQ) (see table 2 & 3 and figure 2). Figure 2 shows visually that only a few samples results for DR CALUX® in human, buffalo and horse plasma samples are above normal population levels suggesting that such bioassay screening tools are very helpful to focus on these higher polluted cases for further investigations:
Table 2 and figure 2
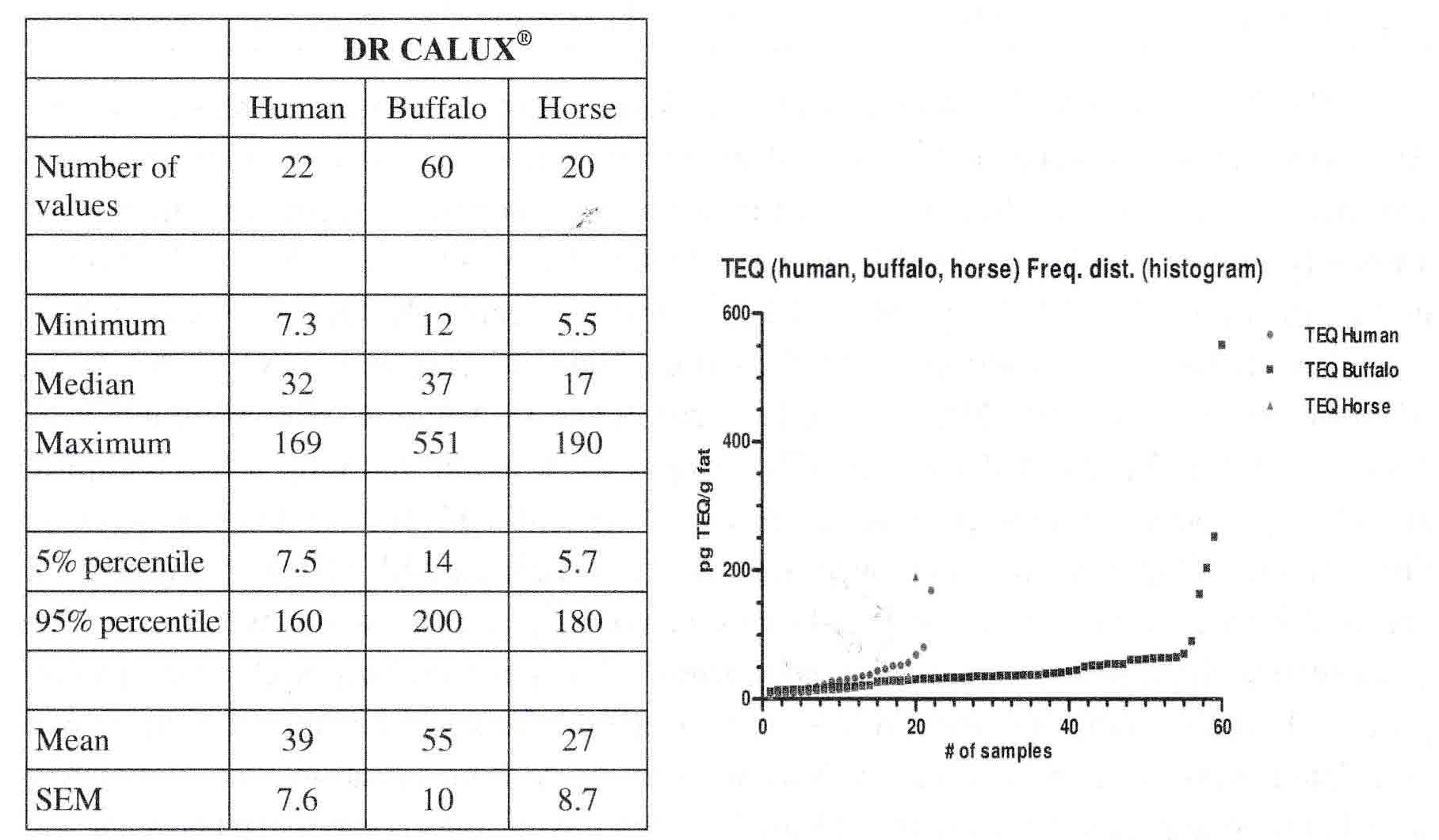 |
Minimum, median, maximum, mean, 95% and 5% percentile value of the PCDD/PCDF/dl-PCB/dl-compounds-TEQ analysed by DR CALUX® from human, buffalo and horse plasma samples in several locations in the Campania region, Italy (e.g. Avellino, Salerno, Caserta and Napoli area).
Table 3. Range, mean and LOD results for the different CALUX® bioassays from human, buffalo, horse, dogs and sheep plasma samples from several areas in Italian’s Campania Region
 |
The 3rd case (USA, 2016) involved horse plasma samples collected across the USA: Persistent organic pollutants are associated with metabolic syndrome and other endocrine abnormalities in humans, In horses, such abnormalities in insulin regulation, increased adiposity and laminitis can lead to Equine Metabolic Syndrome (EMS). Therefore, in our study here Ah (TEQ; by DR CALUX®) and estrogenic (EEQ, by ER CALUX®) receptors analysis were used as biomarker-of-exposure in plasma of 288 horses from 33 farms (phenotyped for EMS) (see table 4 and figure 3). The with increasing analysis results in figure 3 presented DR CALUX® results show again that only a few samples are clearly above normal population levels (“vertical end of the hockey stick”). Furthermore, TEQ concentrations were negatively associated with plasma fat extracted and positively associated with the Welsh pony breed. EEQ concentrations were positively associated with pregnancy status and being sampled in summer. Serum glucose, insulin post oral sugar challenge, insulin and non-esterified fatty acid concentrations were associated with EEQ concentration. Serum triglyceride concentration was associated with TEQ concentration. For more info, please let US refer to the already submitted paper [6],
Table 4 and figure 3. Blood plasma of 288 horses from 32 farms phenotyped for Equine metabolic syndrome (EMS) analysed by DR CALUX® (for dioxin-like compounds)
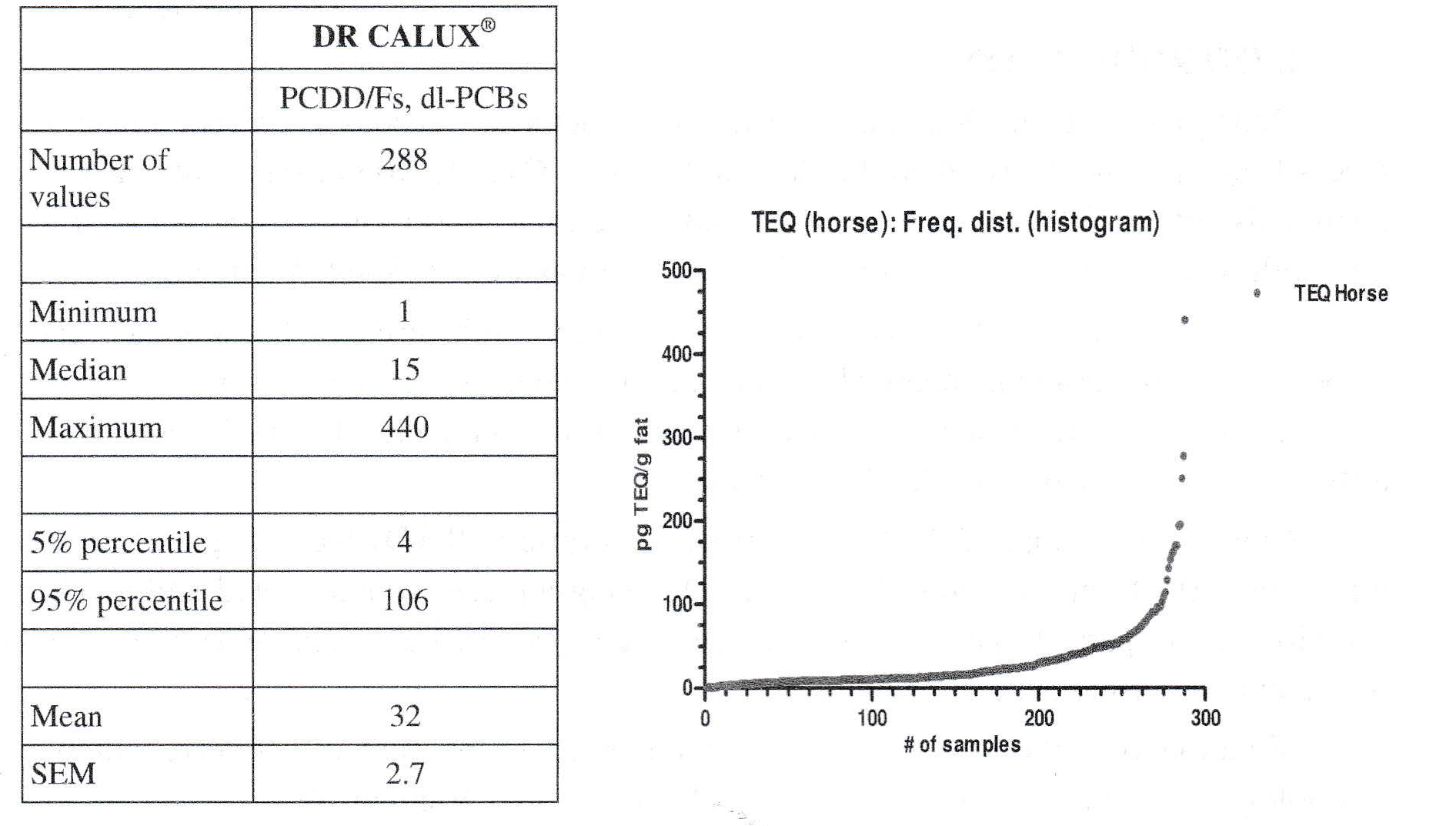 |
In the present study, we present three cases of blood plasma monitoring of humans and domestic animals in in Germany, Italy and USA by using a panel of CALUX® bioassays for stable AhR compounds (such as dioxins), labile AhR compounds (such as PAHs), genotoxicity, oxidative stress and endocrine disrupting chemicals. These biomonitoring results of these relevant mode of actions (MoA) show that cell- and effect-based testing (such as CALUX® panel) of mixtures of known toxic compounds and yet unknown pollutants in blood in humans and domestic animals is an important tool to separate the bulk of unpolluted samples from the few samples exceeding background levels.
REFERENCES
1. Behnisch P.A (2005); Rapid Methods for biological and chemical contaminants in food and feed. Wageningen Academic Publishers, pp.303-318.
2. Behnisch PA (2005). Food. Issue 1, pp.13-17.
3. Vafeiadi M, Agramunt s, Pedersen M et al. (2014); Epidemiology, 25(2): 215-224.
4. Brouwers MM, Besselink H, Bretveld RW et al. (2011). Environ. Int., 37:557-564.
5. Aylward LL, Lakind JS, Hays SM. et al. (2008). J. Toxicol. Enivron. Health Pt. A.71 (22): 1499-1508.
6. Durward-Akhurst SA, Schultz NS, Norton EM. et al. (2018); submitted, Environ. Int.
Behnisch PA1; Besselink H1; Malonek L2; Litnone A3; Pizzokinte A3; Pierri A3 Ferro A3; Gallo A3; Buonerba C, Pierri B4; Di Stasio A3; Cerino P3 Durward-Akhurst SA5; Schultz NE5; Norton EM5; Rendahl AK6
Geor RJ7; Mickelson JR8; McCue ME5; Brouwer A1,9
1. BioDetection Systems BV (BDS), Science Park 406, 1098 XH Amsterdam, the Netherlands, peter@bds.nl
2. Interessengemeinschaft Endlager Mensch e.v., Buchenstrasse 14, 47475 Kamp-Lintfort, Germany
3. Istituto Zooprofilattico Sperimentale del Mezzogiomo, Portici, Italy
4. University of Salerno, Salerno, Italy
5. Department of Veterinary Population Medicine, Veterinary Medical Center, 1365 Gortner Avenue, St. Paul, MN, 55108, USA
6. College of Veterinary Medicine, 1988 Fitch Avenue, St. Paul, 55108, USA
7. College of Sciences, B2.13, Science Tower B, Massey University, Turitea, New Zealand
8. Department of Veterinary and Biomedical Sciences, 301 Veterinary Science Building, 1971 Commonwealth Avenue, St. Paul, 55108, USA
9. Department of Ecological Sciences, VU University of Amsterdam, De Boelelaan 1089, 1081 HV, The Netherlands

































Comment