SUMMARY
Chlorinated dioxins are broadly contaminating subsurface environments such as aquifers and soils in Vietnam and elsewhere. Bioaccumulation within the food chain and their strong toxicity make dioxins a contamination of high concern. Natural transformation was negligible within the last 50 years and treatment of sources is urgently necessary for public health and economic reasons.
The only economically feasible approach for the treatment of large areas with underground contaminations are biotechnological applications using the metabolic capabilities of bacteria. Microbiological, biochemical and genomic studies in the last 25 years have firmly established that the only feasible biotechnological process is the anaerobic treatment with Dehalococcoides- containing mixed cultures. Only if microorganisms specifically profit from the transformation of dioxins, a sustainable biotechnological process can be established. This is the case with Dehalococcoides species which use dioxins as an electron acceptor for respiration. The organisms grow under strictly anoxic conditions and spread within the contaminated subsurface. The application of Dehalococcoides-containing cultures is safe and commercially applied at many different sites worldwide, mostly for the treatment of chlorinated ethenes. Several internationally-connected scientific groups in Vietnam have experience with anaerobic cultivation and are ready for applying the technology.
I. CHEMICAL PERSPECTIVE
Dioxins are highly oxidized chemicals, meaning that they are electron-deficient, due to the high electron-removing (-I effect) effect of halogen substituents. The natural approach to transform highly oxidized molecules should be the addition of electrons, i.e. a reductive treatment under anoxic conditions. This is known since long and documented in the scientific literature (Lynam et al. 1998). Neither the oxidative transformation of highly oxidized dioxins nor the reductive treatment of highly reduced compounds (e.g. oil) is electrochemically favorable.
II. MICROBIAL PERSPECTIVE; BACTERIA NEED TO BENEFIT FROM DIOXIN TRANSFORM ATION
2.1. Biotechnology
Almost all successful processes in biotechnology use an approach in which the organisms that perform the target reaction profit from the reaction. We here have todifferentiate between conditions enriching for the key organisms (\"enrichment\") and conditions that disfavor the growth of competing organisms (\"selective inhibition\"). Enrichment can be achieved by using the target chemical as food (as electron donor, electron acceptor or C-, N-, P-donor). To give some examples: In rice or shrimp production, optimal conditions are set to promote growth of rice or shrimps, respectively. Primarily, appropriate feeding conditions are applied (light, nutrients, food) and only secondarily competing organisms are inhibited (antibiotics, pesticides). The same is true for microbial biotechnological processes such as biogas production, bioethanol production, waste water treatment, yogurt production or copper bioleaching. In waste water treatment, for example, bacteria grow by oxidizing the carbon substrates to co? and water. By doing this they grow to extremely high cell numbers and the waste water treatment plant can work efficiently. In biogas plants methane-forming archaea gain energy from the production of methane under anaerobic conditions and grow to very high numbers making biogas production an efficient self-sustaining process. Also other contributing microorganisms in biogas plants (e.g. polymer hydrolyzing, fermenting, acetogenic bacteria) benefit from their activity. More generally speaking: efficient microbial processes can only occur if the key bacteria benefit from the process.
For dioxin transformation in Vietnam this means: efficient microbial removal of dioxins from soil and groundwater in Vietnam can only occur if a treatment option is used in which the catalyzing bacteria selectively profit from dioxin conversion. Only then the key bacteria grow to reasonable cell numbers, distribute throughout the contaminated site and remove dioxins.
2.2. Bacteria of the genus Dehalococcoides
Bacteria of the genus Dehalococcoides are known to transform dioxins since 2003 when we showed that Dehalococcoides strain CBDB1 dechlorinates many different dioxin congeners (Bunge et al. 2003). The strain also transformed the most toxic congeners including 2,3,7,8-tetrachlorodibenzodioxin (Fig. 7) leading to products with much lower toxicity.
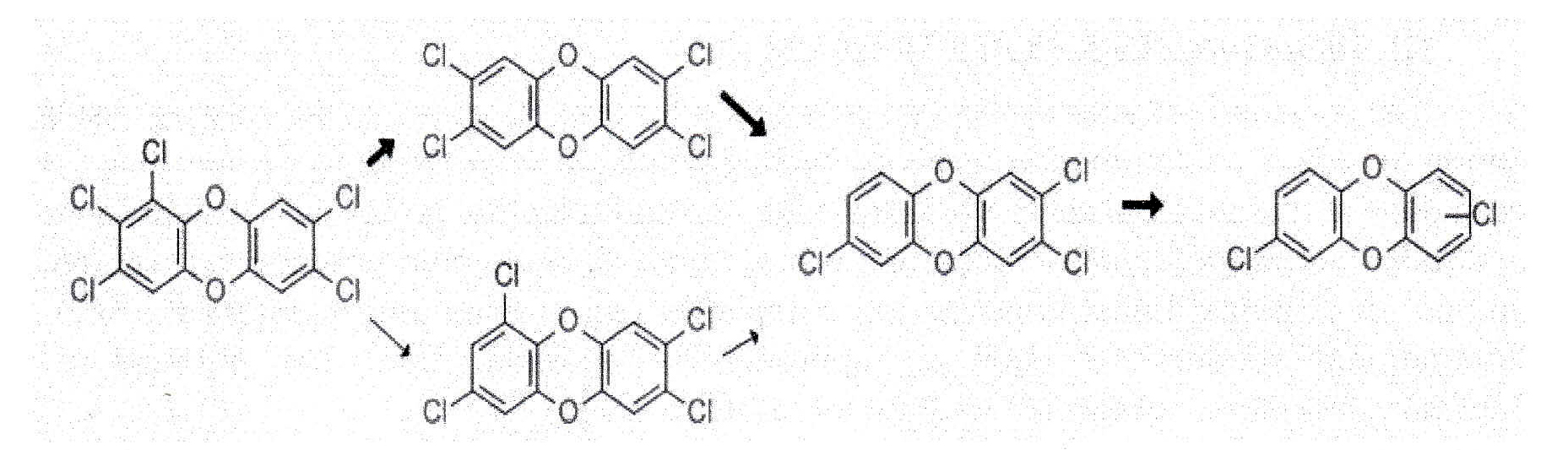 |
Fig. 1. Microbial transformation of dioxins halogenated on both lings by Dehalococcoides strain CBDB1. The strain detoxifies the most toxic dioxins to less halogenated and less toxic structures (Bunge et al, 2003)
These results confirmed earlier findings that the anaerobic transformation of dioxins by mixed cultures is feasible and efficient and all is well documented in the scientific literature (Beurskens et al, 1995; Barkovskii and Adriaens, 1996; Ballerstedt et al, 1997; Bunge et al, 2001; Vargas et al, 2002). Later, many studied documented the presence and distribution of Dehalococcoides strains in such anaerobic mixed cultures and argued that the Dehalococcoides strains in the cultures are the key organisms responsible for dioxin transformation (Ballerstedt et al, 2004; Ahn et al, 2005; Hiraishi et al, 2005; Yoshida et al, 2005; Ahn et al, 2007; Ewald et al, 2007; Bunge et al, 2008; Hiraishi, 2008; Bunge and Lechner, 2009; Narihiro et al, 2010; Dam et al, 2017). Efficient anaerobic transformation of dioxins requires the presence of Dehalococcoides species.
Dehalococcoides use halogenated dioxins for energy production and therefore benefit from the reactions (see Box 1 on the next page for biochemical details). They grow if halogenated dioxins are present and stop growing if halogenated compounds are not available. The important aspect for the application in the field is that Dehalococcoides bacteria distribute in the subsurface with the groundwater flow wherever dioxins are present and no mixing is necessary. When no halogenated compounds are available they will not further grow. With this Dehalococcoides represent the optimal agent for underground remediation of dioxin contaminations. Pathogenicity has never been observed and is unlikely because cells die within one second when exposed to oxygen (Adrian et al, 2000). For the bioaugmentation with Dehalococcoides this gives the challenge to remove oxygen from the underground. However, this can be achieved by addition of electron-rich substrates such as polylactate or vegetable oil which distribute together with the halogenated dioxins and provide electrons for anaerobicity and dioxin reductive dehalogenation (Steffan and Schaefer, 2016).
III. PERSPECIVES FOR VIETNAM
The contaminated sites in Vietnam now persist for over 50 years, but the only promising option to reduce contamination levels by bioaugmentation with Dehalococcoides has not been done at reasonable scale to my knowledge. Several scientific groups in Vietnam know to cultivate Dehalococcoides-containmg mixed consortia and could produce the required amount of cultures. Local construction companies can prepare and maintain the sites. International support for such an approach is available from the international \"Dehalococcoides-community\" (Adrian and Loffler, 2016).
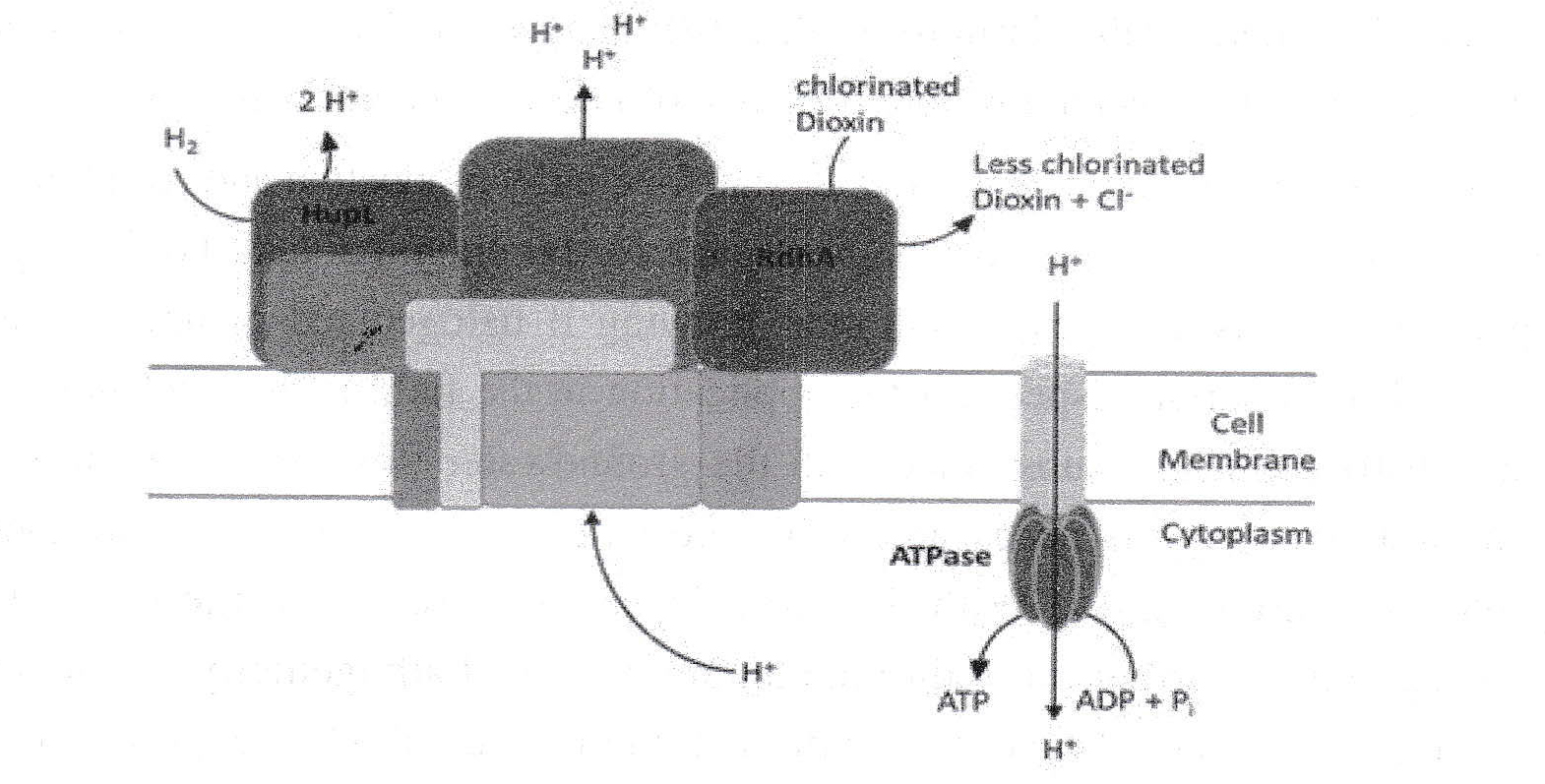
| Box 1: Biochemical background Microorganisms need sources of carbon, nitrogen, phosphor etc., but most of the substrates (~90%) are used for energy production. Respiring organisms need an electron donor and an electron acceptor for energy conservation. Anaerobic bacteria in soil or groundwater use small molecules as electron donor and an electron acceptor other than oxygen. Dehalococcoides species use chlorinated dioxins as an electron acceptor in an anaerobic respiration. By this respiration (Fig. 2) a proton gradient is generated across the cell membrane which is used for ATP regeneration by an ATPase. We are investigating the biochemical background in detail in our laboratory (Kublik et al, 2016; Seidel et al, 2018). Biochemical peculiarities in Dehalococcoides (missing quinones, missing cytochromes, monoderm cell envelope, organization of all electron transferring subunits in one single protein complex) might confer the capacity to catalyze exceptional reduction reactions
Fig. 2. Sketch of energy conservation by membrane-bound proteins in Dehalococcoides using halogenated dioxins as electron acceptor. The electron transfer from hydrogen to dioxins is driving the export of protons (H+) to the extracellular space. The ATPase uses the energy of the electrochemical gradient for ATP regeneration. Hydrogen is available in all anaerobic environments and is usually not limiting. |
* Aerobic microbial transformation of dioxins is not efficient:
Aerobic microbial transformation of dioxins has been reported in the literature. Indeed aerobic transformation of dioxins can occur as the result of the enzymatic effect of oxygenases. In the presence of air (oxygen) oxygenases produce reactive oxygen species that can react with dioxins. With this, dioxins can be activated and successively used as a carbon and electron source. However, due to the low concentration of dioxins in environmental samples dioxins cannot be a significant source of carbon and electrons for microorganisms compared to other nutrient sources in soil or groundwater. In that way, dioxin oxygenation is more a side reaction of other oxygenations. Never has been demonstrated an enrichment of bacteria on the basis of their capacity to oxygenate dioxins.* Aerobic microbial transformation of dioxins is not efficient:
* Application: To aerobically treat dioxin contaminations it is necessary to dig out contaminated soil or pump out contaminated groundwater and treat off situ. For this the soil or groundwater needs to be stored on a separate site, be aerated for several weeks and carefully monitored. This procedure does not work in the following cases: i) widespread contaminations; ii) contaminations that are deep in the ground; iii) contaminations below cities, industrial sites or important infrastructure that cannot be removed, iv) if financial resources are limited. In Vietnam one or more of these conditions apply for almost all contaminated sites. Therefore, anaerobic treatment should be preferred over aerobic treatment.
Lorenz Adrian1
REFERENCES
1. Adrian L, Loffler F (2016) Organohalide-Respiring Bacteria, vol 1. Springer-Verlag, Berlin Heidelberg, doi: 10.1007/978-3-662-49875-0
2. Adrian L, Szewzyk u, Wecke J, Gorisch H (2000) Bacterial dehalorespiration with chlorinated benzenes. Nature 408 (6812):580-583. doi:l0.1038/35046063
3. Ahn Y-B, Haggbiom MM, Kerkhof LJ (2007) Comparison of anaerobic microbial communities from estuarine sediments amended with halogenated compounds to enhance dechlorination of 1,2,3,4-tetrachlorodibenzo-p-dioxin. FEMS Microbiol Ecol 61 (2):362-371
4. Ahn YB, Haggblom MM, Fennell DE (2005) Co-amendment with halogenated compounds enhances anaerobic microbial dechlorination of 1,2,3,4-tetrachlorodibenzo-p-dioxin and 1,2,3,4- tetrachlorodibenzofuran in estuarine sediments. Environ Toxicol Chem 24 (ll):2775-2784
5. Ballerstedt H, Hantke J, Bunge M, Werner B, Gerritse J, Andreesen JR, Lechner Ư (2004) Properties of a trichlorodibenzo-p-dioxin-dechlorinating mixed culture with a Dehalococcoides as putative dechlorinating species. FEMS Microbiol Ecol 47 (2):223-234
6. Ballerstedt H, Kraus A, Lechner u (1997) Reductive dechlorination of 1,2,3,4- tetrachlorodibenzo-p-dioxin and its products by anaerobic mixed cultures from Saale river sediment. Environ Sci Technol 31 (6): 1749-1753. doi:10. IO21/es960737h
7. Barkovskii AL, Adriaens P (1996) Microbial dechlorination of historically present and freshly spiked chlorinated dioxins and diversity of dioxin- dechlorinating populations. Appl Envứon Microbiol 62 (12):4556-4562
8. Beurskens JEM, Toussaint M, de Wolf J, van der Steen JMD, Slot PC, Commandeur LCM, Parsons JR (1995) Dehalogenation of chlorinated dioxins by an anaerobic microbial consortium from sediment. Environ Toxicol Chem 14 (6):939-943
9. Bunge M, Adrian L, Kraus A, Opel M, Lorenz w, Andreesen J, Gorisch H, Lechner u (2003) Reductive dehalogenation of chlorinated dioxins by an anaerobic bacterium. Nature 421 (6921 ):357-360. doi: 10.1038/nature01237
10. Bunge M, Ballerstedt H, Lechner U (2001) Regiospecific dechlorination of spiked tetra-and trichlorodibenzo-p-dioxins by anaerobic bacteria from PCDD/F-contaminated Spittelwasser sediments. Chemosphere 43 (4-7):675-681. doi:http://dx.doi.org/10.1016/S0045- 6535(00)00420-3
11. Bunge M, Lechner U (2009) Anaerobic reductive dehalogenation of polychlorinated dioxins. Appl Microbiol Biotechnol 84 (3):429-444. doi: 10.1007/s00253-009-2084-7
12. Bunge M, Wagner A, Fischer M, Andreesen JR, Lechner U (2008) Enrichment of a dioxin-dehalogenating Dehalococcoides species in two-liquid phase cultures. Environ Microbiol 10 (10):2670-2683. doklO.l 111/j. 1462-2920.2008.01688.x
13. Dam HT, Vollmers J, Raster A-K, Haggblom MM (2017) Reconstructed genomes of novel Dehalococcoides mccartyi strains from 1,2,3,4-tetrachlorodibenzo-p-dioxin-dechlorinating enrichment cultures reveal divergent reductive dehalogenase gene profiles. FEMS Microbiol Ecokfix 151 -fix 151. doi: 10.1093/femsec/fix 151
14. Ewald EM, Wagner A, Nijenhuis I, Richnow HH, Lechner U (2007) Microbial dehalogenation of trichlorinated dibenzo-p-dioxins by a Dehalococcoides-containing mixed culture is coupled to carbon Isotope fractionation. Environ Sci Technol 41 (22):7744-7751
15. Hiraishi A (2008) Biodiversity of dehalorespiring bacteria with special emphasis on polychlorinated biphenyl/dioxin dechlorinators. Microbes and Environments 23 (1): 1 -12
16. Hiraishi A, Sakamaki N, Miyakoda H, Maruyama T, Kato K, Futamata H (2005) Estimation of \"Dehalococcoides\" Populations in Lake Sediment Contaminated with Low Levels of Polychlorinated Dioxins. Microbes and Environments 20 (4):216-226
17. Kublik A, Deobald D, Hartwig S, Schiffmann CL, Andrades A, von Bergen M, Sawers RG, Adrian L (2016) Identification of a multi-protein reductive dehalogenase complex in Dehalococcoides mccartyi strain CBDB1 suggests a protein-dependent respiratory electron transport chain obviating quinone involvement. Environ Microbiol 18 (9):3044—3056. doi: 10.1111 /1462-2920.13200
18. Lynam MM, Kuty M, Damborsky J, Koca J, Adriaens p (1998) Molecular orbital calculations to describe microbial reductive dechlorination of polychlorinated dioxins. Environ Toxicol Chem 17 (6):988-997. doi: 10.1002/etc.5620170603
19. Narihiro T, Kaiya s, Futamata H, Hiraishi A (2010) Removal of polychlorinated dioxins by semi-aerobic fed-batch composting with biostimulation of “Dehalococcoides”. Journal of Bioscience and Bioengineering 109 (3):249-256. doi:10.1016/j.jbiosc.2009.08.498
20. Seidel K, Ktihne J, Adrian L (2018) The complexome of Dehalococcoides mccartyi reveals its organohalide respiration-complex is modular. Front Microbiol 9:1130. doi: 10.3389/ fmicb.2018.01130
21. Steffan RJ, Schaefer CE (2016) Current and future bioremediation applications: bioremediation from a practical and regulatory perspective. In: Adrian L, Loffler FE (eds) Organohalide-Respiring Bacteria. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 517-540. doi: 10.1007/978-3-662-49875-0_22
22. Vargas c, Fennell DE, Hăggblom MM (2002) Anaerobic reductive dechlorination of chlorinated dioxins in estuarine sediments. Appl Microbiol Biotechnol 57 (5-6):786-790
23. Yoshida N, Takahashi N, Hiraishi A (2005) Phylogenetic characterization of a polychlorinated-dioxin-dechlorinating microbial community by use of microcosm studies. Appl Environ Microbiol 71 (8):4325-4334
1. Helmholtz Centre for Environmental Research - UFZ, Department Isotope Biogeochemistry, Working group Geobiochemistry, Leipzig & Chair of Geobiotechnology, Technische Universităt Berlin, Germany

































Comment